This is the energy required to ionise a single He ion. This is the energy required to remove an electron from an atom scaled up to 1 mole.

Tin Electron Affinity Electronegativity Ionization Energy Of Tin Nuclear Power
4 Determine energy for one mole of photons.

Ionization energy of tin in kj/mol. E hc λ. Just make sure that the units for c and λ are the same. Melting Point Celsius scale 631.
First Ionization Energy of Tin is 73438 eV. Lattice enthalpy of MOs Hl -2315 kJmol. Arrange the elements in decreasing order of first ionization energy.
The first ionization potential of Na Mg and Si are 496 737 and 786 kJ mol-1 respectively. Lattice enthalpy of MOs Hl -2315 kJmol Bond dissociation enthalpy of O2g 498 kJmol First electron affinity of O -141 kJmol Second electron affinity of O 744 kJmol Enthalpy of sublimation of M 184 kJmol First ionization energy of M 318 kJmol Standard enthalpy of formation of MOs Hf -182 kJmol. Ionisation Energies and electron affinity.
In Indium- The ionization energy is 5583 kJmol Sn Tin- The ionization energy is 7086 kJmol The elements according to the given ionization energy are written below a 558 kJmol- This. These tables list values of molar ionization energies measured in kJmol1. A representation of the atomic spectrum of tin.
Ionization energy kJmol- Get the answer to this question and access more number of related questions that are tailored for students. First electron affinity of O -141 kJmol. 124 rows These tables list values of molar ionization energies measured in kJmol 1This is the.
To find the energy required to ionise a mole of ions you need to multiply by The Avogadro Constant which is 602 1023xmol1. First Ionization level IE1. 39756 x 10 19 J 6022 x 10 23 mol 1 2394 kJmol.
Ionization energy is minimal energy needed to detach the electron from the atom or molecule. Ionization Energy E h v h v λ 66 10 34 3 10 8 242 10 9 198 242 10 17 818 10 15 J a t o m Ehvfrachvlambda frac66times 10-34times 3times 108242times 10-9frac198242times 1017818times 1015Jatom E h v λ h v 2 4 2 1 0 9 6. Thermal Conductivity Wm K 24.
Bond dissociation enthalpy of O2g 498 kJmol. On Earth the ionization energy of atomic hydrogen is 1312 kJmol. X energy X e.
To see an ionization energy example at play lets look at sodium. Ionization energy is given a number of symbols including I E and IE. Ionization energy is measured in kilojoules per mole kJ mol-1 or electronvolts per atom eV 1.
Second electron affinity of O 744 kJmol. 6 1 0 3 4 3 1 0 8 2. This is the energy.
Identify the elements X and Y using the ionization energy values given below. Click to see full answer. First ionization energy refers to the energy required to remove an electron from an electrically neutral atom in the gas phase.
Specific Heat Jg K 021. For this ionization energy example Na stands for sodium and e- is the electron that is removed from the sodium atom. It is quantitatively expressed as Xg energy X g e.
This can be explained by noting that the outermost or highest energy electron on alithium atom is in the 2sorbital. Boiling Point Celsius scale 1950. NCERT Solutions For Class 12.
Second Ionization level IE2. Enthalpy of sublimation of M 184 kJmol. IE 872 1021 602 1023 5250xkJmol.
119 rows The chart shows Ionization Energies in kJmol. Nag Nag e-IE1 496 kJmol. Atomic 1 2 3 4.
Heat of Vaporization kJmol 7714. Ionization energy or ionisation energy is the energy required to remove an electron from a gaseous atom or ion. The electron affinity of tin is 1073 kJ mol 1The ionisation energies of tin are given below.
To find the energy required to ionise a mole of ions you need to multiply by The Avogadro Constant which is 6021023mol1. Electron Affinity of Tin is 1073 kJmol. The ionization potential of Al will be closer to asked Sep 26 2020 in Periodic Classification of Elements by Manish01 476k points.
Rank from highest to lowest first ionization energy. This is the energy required to ionise a single He ion. Third Ionization level IE3.
Electron Affinity kJmol 1032. In physics and chemistry ionization energy American English spelling or ionisation energy British English spelling is the minimum amount of energy required to remove the most loosely bound electron of an isolated neutral gaseous atom or molecule. Electronegativity Pauling scale 205.
Calculate the ionization energy of sodium in kJ mol 1. The difference in energy between these levels will represent the ionisation energy. 20 rows Electron Affinity and Electronegativity of Tin.
Where X is any atom or molecule X is the resultant ion when the original. Because the electron in a 2sorbital isalready at a higher energy than the electrons in a 1sorbital it takes less energyto remove this electron from the atom. If you wished to do a direct calculation you could use this equation.
1st Ionization Energy eV 864. Ionization energy also called ionization potential is the energy necessary to remove an electron from the neutral atom. Ionization energy is also referred to as ionisation energy.
Calculate the second ionization energy of the metal M Hion2 in kJmol using the following data. Nag Na2g e IE2 4560 kJmol. Heat of Fusion kJmol 1987.
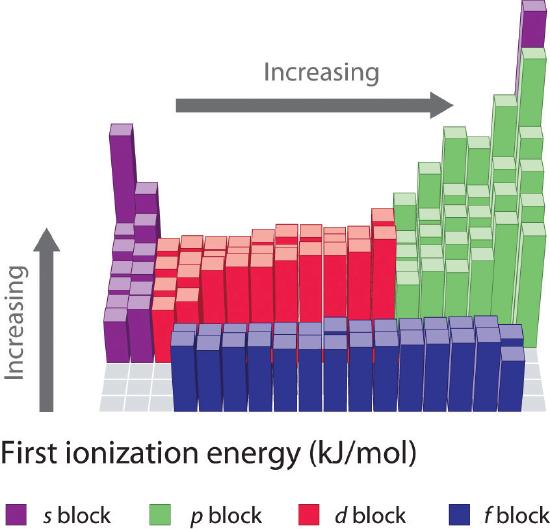
7 4 Ionization Energy Chemistry Libretexts
Tidak ada komentar