Lead Tin and Alloys Nickel Brasses Nickel-Silvers Copper Bronzes Cupro-Nickels Nickel Copper Alloys Nickel-Chrome Alloys Titanium Silver Graphite Gold and Platinum Contact Metal Corroding Metal Galvanic Corrosion Risk This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given. The present invention provides a galvanic cell having an aluminum anode and a cathode compartment design suitable for carrying out the aqueous electrochemical reaction between solid aluminum metal.
Electrochemical Cells Ck 12 Foundation
In writing the equations it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations.
Tin and aluminum galvanic cell. The corrosion of the base metal is slightly increased by the fastener. Answer 1 of 2. GALVANIC CORROSION - COMPATIBLE METALS CHARTS CORROSION INFORMATION GALVANIC ACTION Revised by TFC.
Tin plating starts to oxidize the moment its exposed to air. Figure 1 Corrosion on a galvanized screw in a coastal location. Unlike gold tin is not a noble metal.
The aluminum electrodes in alkaline aluminum galvanic cells are inhibited against corrosion while the cell is on open circuit or while the cell is being operated at very low discharge current by an inhibitor system comprising an alkali metal citrate plus a lead compound a tin compound or both a lead compound and a tin compound. So a tin-plated contact system requires greater normal forces and a longer contact wipe area to break through this oxide film. The cell in which reduction occurs is called the cathode.
Fortunately aluminum tends to build stable corrosion products which helps minimize corrosion somewhat tin and aluminum are reasonably close in the galvanic series and the anodic surface area is large compared to the cathodic surface area which spreads a limited galvanic current across a larger area. The picture opposite shows a Galvanic cell made from zinc and tin half cells. Partial Galvanic Series Table 31 ASNZS 2312.
Zn2 2e Zns 3 ξ 076V. Als Al3 3e 2 ξ 166V. In the first instance only using metals in combination that are relatively close to each other in terms of galvanic potential would limit or avoid galvanic corrosion.
Whatever your industry whatever your application Orbels custom design and. The cell potential is calculated. Aluminum and Aluminum Alloys Chromium Tin and Tin Plating Silver Chromium Cadmium Plating TinLead Alloys Solder and Plating Graphite.
For the reaction between Cu. Eº cell Eº cathode - Eº anode 034 - - 014 048 V. The galvanic corrosion performance of the different aluminum alloys is quite similar so that changing alloys cannot solve the problem.
A galvanic series applies to a particular electrolyte solution hence for each specific solution which is expected to be encountered for actual use a different order or series will ensue. The galvanic series for metals show that aluminum will be the anode of the galvanic cell in contact with almost all other metals and hence the one which suffers from galvanic corrosion. Issues regarding galvanic corrosion please contact Orbels technical sales or engineering department at 610-829-5000.
Stainless Steel 18 CR 8 Ni active-061. The corrosion of the base metal is not increased by the fastener. Since the copper half-cell is the cathode this is where the reduction occurs and the tin half-cell is the anode where the oxidation occurs our calculation would be.
In this couple the aluminium would act as the anode in an electrochemical cell and be preferentially attacked. There are three conditions that must exist for galvanic corrosion to occur. Tin is oxidized at the anode while silver ion is reduced at the cathodeCalculating Standard Cell Potentials.
The corrosion of the base metal may be considerably increased by the fastener material. In a galvanic couple the metal higher in the series or the smaller represents the. Think about your result.
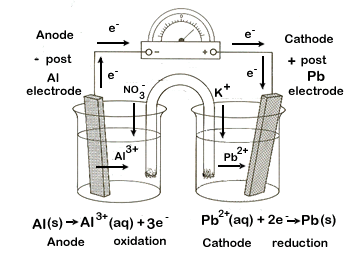
Aluminium bronze Lead Chromium Tin Mild steel cast iron Aluminium alloys Zinc Magnesium Table 1. First there must be two electrochemically dissimilar metals present. A galvanic cell or voltaic cell named after the scientists Luigi Galvani and Alessandro Volta respectively is an electrochemical cell in which an electric current is generated from spontaneous reactions.
Galvanic corrosion some times called dissimilar metal corrosion is the process by which the materials in contact with each other oxidizes or corrodes. Tin is a lower cost alternative than gold and has excellent solderability. The overall reaction in the galvanic cell is the sum of the two half equations.
Note the corrosion of the zinc at the point of contact with the bare steel. The standard cell potential is positive so the reaction is spontaneous as written. A common apparatus generally consists of two different metals each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge.
The zinc half cell has a strip of zinc metal in a beaker containing Zn 2 aq ions. Galvanic cells also known as voltaic cells are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy. Zn2 2e Zns ξ 076V.
How do you calculate standard cell voltage. What does change is the amount of current that can flow having larger plates and all will lower the internal resistance and increase the avail. The voltage is controlled by the relative potential of the materials used so no matter how much you use it will not change the voltage.
. The tin half cell has a strip of tin metal in a beaker containing Sn 2 ions. Galvanic corrosion can be prevented by a variety of means.
Chemistry 30 Electrochemistry Calculating Voltage Of Electrochemical Cells
Tidak ada komentar