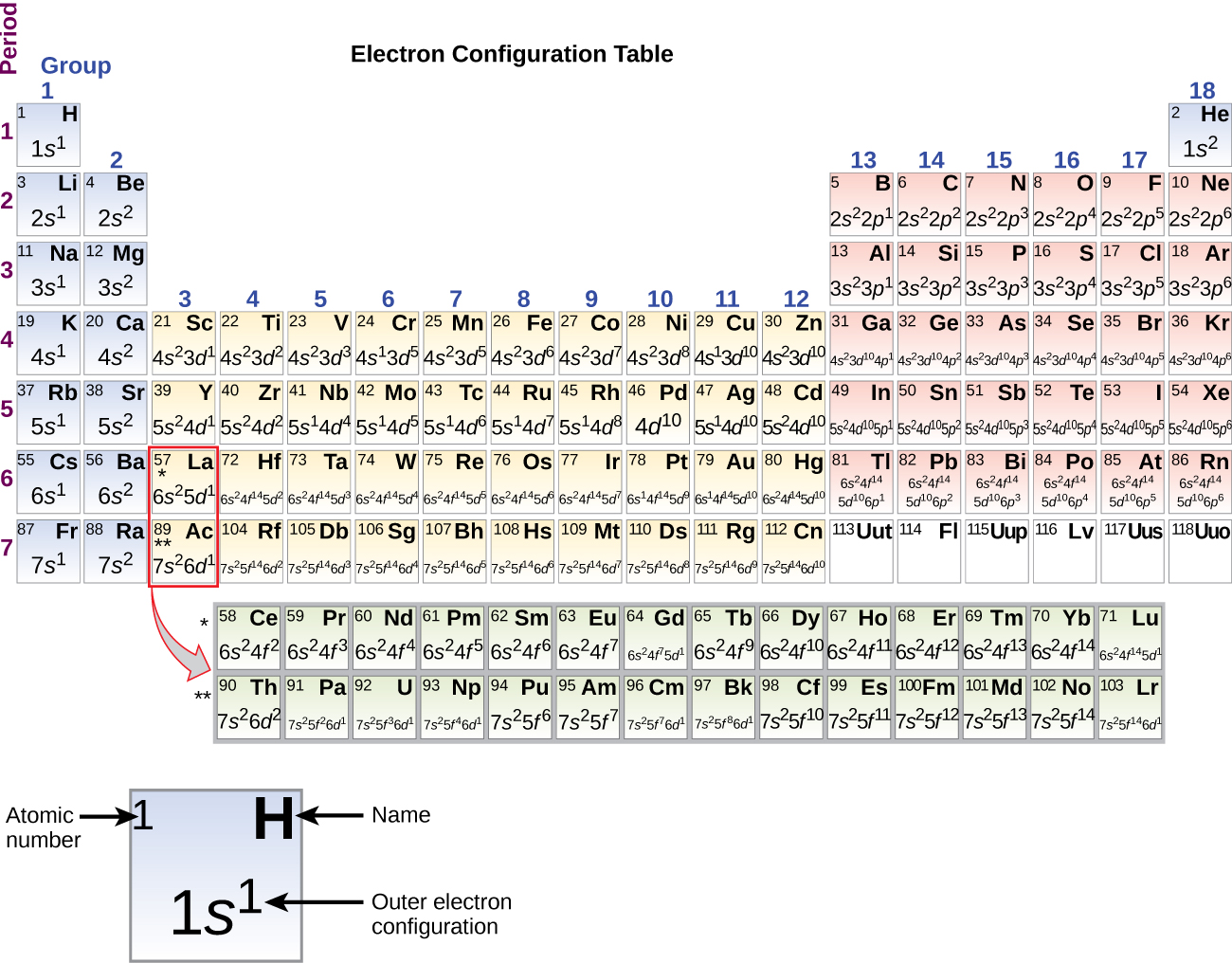
Electronic configuration of chromium is Ar 3d 5 4s 1 NOT Ar 3d 4 4s 2. The electron configuration for titanium is 1s22s22p63s23p63d24s2 according to the Jefferson Lab website.
3 1 Electron Configurations Chemistry Libretexts
Electrons per Energy Level.
Electronic configuration of tin a level. Tin atomic radius is. Electron configuration of Tellurium Te Kr 4d 10 5s 2 5p 4. We know atoms have energy levels.
Nevertheless check the complete configuration and other interesting facts about Tin that most people dont know. For example the electron configuration of the neon atom is 1s2 2s2 2p6 using the notation explained below. Tin Sn Electron Configuration.
Lets look at building up the electronic arrangement electron configuration from hydrogen Z 1 as far as krypton Z 36. The concept of electronic configuration has replaced the older concept of valency and valence electrons. Atomic Structure of Tin.
Electron configuration of Tin Sn Kr 4d 10 5s 2 5p 2. Its electron configuration is. Kr 4d 10 5s 2 5p 2.
Electrons orbit the nucleus in energy levels which are also called shells. 2 8 18 18 4. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 Ni2.
Even though there is repulsion between negatively charged. 1s 2 2s 2 2p 6 3s 2 3p 6 4s OR. A 2p electron is in the second shell and therefore has an energy corresponding to n 2.
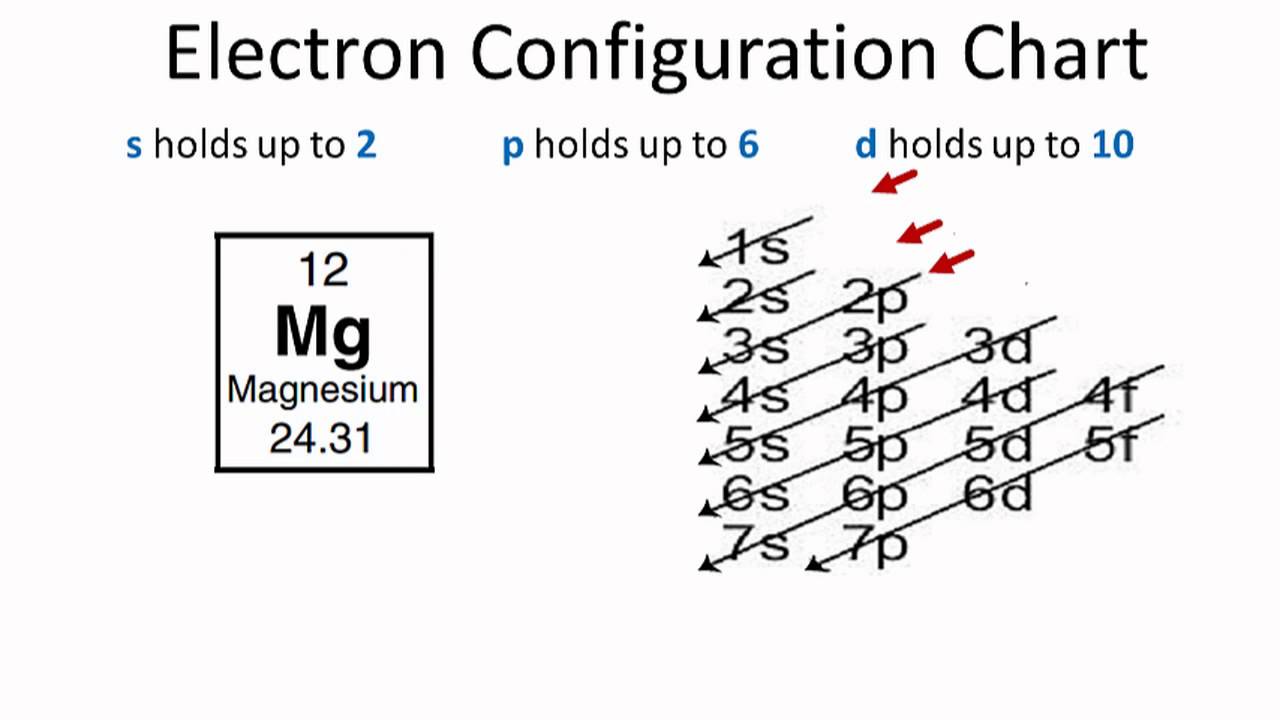
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f. Where do you find energy levels. The arrangement of electrons within an atom is called the electronic configuration and the electrons are filled up according to the energy of the levels as.
P3- ions are no. Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information. Indium tin antimony.
Four electrons in a p subshell. Electronic configuration of d block elements. 1s2 2s2 2p6 3s2 3p6 3d8 To shoe-horn three extra electrons onto a phosphorous atom would require a lot of energy.
Tin atoms have 50 electrons and the shell structure is 2818184. Find out more. Energy Levels Orbital Diagrams Electron Config Noble Gas - Google Slides.
Tin Overview Tin Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s2 4 d10 5 p2 Abbreviated Electron Configuration Kr 4d10 5s2 5p2 Sources. 1s2 2s2 2p6 3s2 3p6 Sn4. Answer 1 of 4.
The electron configuration for tin is 1s22s22p63s23p64s23d104p65s24d105p2. These are a bit different to other elements so I. And electrons are being added to the s subshell of the fourth energy level that is electrons are being added to fill the 4s subshell which can house a maximum of 2 electrons.
P3- assuming it actually exists. The principal quantum number indicates the energy level of a particular shell but also indicates the energy of the electrons in that shell. Complete ground state electronic configuration for the Tin atom Unabbreviated electronic configuration.
N atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. By default electrons are found in the lowest energy level possible close. Learn the basics about Energy Levels and Electron Configuration.
Keep in mind electron configurations are most stable when they are filled or half-filled. Similar electron configurations within a group of the Periodic Table can be emphasised with a simpler representation in terms of. In the case of Tin the abbreviated electron configuration is Kr 4d10 5s2 5p2.
Two electrons from 5p and two electrons from 5s. Kr 4d10 5s2 5p2. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 2.
The elements 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. You can write the full electron configuration in terms of subshells. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2.
Tin Sn Electron Configuration. The elements in which the electron enters in n -1d orbital are called d-block elements. And thus 50 electrons must be distributed according to the usual aufbau schemeTin lies in Group 14 and thus should have a similar electronic configuration to carbon.
5p 2 and the term symbol is 3 P 0. 1s22s22p63s23p64s23d104p64d105s25p2 For tin Z50. Electrons fill the lowest energy level first this means it is generally easy to predict how the electrons will fill the orbitals it gets more complicated with the transition metals.
In this video we look at how to work out the electron configuration of ions of the elements in the d block. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. Rn 5f 14 6d 10 7s 2 7p 6.
The periodic table shows us energy levels 1 7. The ground state electron configuration of ground state gaseous neutral tin is Kr. Electron configuration of Antimony Sb Kr 4d 10 5s 2 5p 3.
For example lithiums electron configuration is 1s 2 2s 1. Going back to the above example Lithium is 1s 2 2s 1 1s has 2 electrons 2s has 1 electron. How do you recognize electron configuration.
Which electrons are lost in the formation of the Sn4 cation. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Full electron configuration of tin.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. These are placed in the middle of the periodic table between s and p-block elements due to their chemical behavior like boing point melting by specific heat density ionization energy bonding etcThe general electronic configuration of valence electron of. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 3.
The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. Unabbreviated electronic configuration of neutral Tin. We start at lithium on the periodic table and we see it is in the second row and the first column of the s-block so its electron.
The order of increasing energy of orbitals as shown below is backed by experimental data. 2 8 18 18 5.
